Filtration Tips
Sterile filtration
When to sterile filter? Common wisdom suggests that wines should be sterile filtered whenever the potential for post-packaging problems is anything more than remote, or if a winemaker simply wants a quality guarantee. This could include the presence of fermentable sugars, but may also extend to malic acid or even the likelihood of physical precipitation of some sort. There is also an element of security that is provided by sterile filtration, which appeals to some producers, regardless of the wine’s prior stability. In any case, sterile filtration is very much the norm these days. Consider, seven years ago around 20% of wine was sterile filtered, whilst these days it’s around 80%.
Nominal/absolute/LRV
These terms were discussed in a previous article (Bowyer, 2015). They refer to the efficiency of a filter, but are dependent on the reference level applied. For example, some manufacturers refer to a filter with a beta ratio of 1000 as absolute. Strictly speaking, a filter should have a beta ratio of 5000 or greater to be designated as absolute. The term nominal has no specific meaning beyond “approximately”. These designators are only really used when describing particulate filters. Most wine depth filters, like sheets and lenticulars, are nominal, although production is very tightly controlled to ensure consistency and accuracy.
The terms “absolute” and “nominal” do not really apply to membranes, for the simple reason that these terms refer to a particulate testing standard, not microorganisms. Additionally, an absolute rated filter still falls well short of a good membrane in terms of filtration efficiency: The efficiency of membranes is typically at least 4 orders of magnitude greater than a b5000 depth filter, hence the term “Log Reduction Value” (LRV) is used for preference. Further, whilst absolute rated depth (particulate) filters are tested against inanimate particulates of defined size, membranes are challenged with microorganisms, which are crafty beasties that can deform to fit through smaller pore sizes. Any good membrane will quote LRV’s for several organisms.
Typically, a table will be provided by the membrane manufacturer on the filter’s technical data sheet specifying the performance of that membrane with respect to several test organisms. If no data table is provided, or if the data table is limited in the information it provides, be wary, as the user will have no easy method of gauging the suitability of the membrane in question for the task at hand. Additionally, there may be a reason as to why specific retention data are not quoted.
The data table for the Parker-domnick hunter Bevpor PH is provided in Figure 1. Note that retention data are provided for each organism at suitable membrane porosities: 0.45 um, 0.65 um and 1.2 um. For wine, relevant organisms are Saccharomyces, Lactobacillus, Acetobacter, and Brettanomyces (amongst others), hence data for all of these organisms are quoted. The most common wine membrane porosities used in Australia in bottling lines are 0.45um (final) and 0.65um (prefilter), hence these membrane performances are specifically quoted for all organisms.
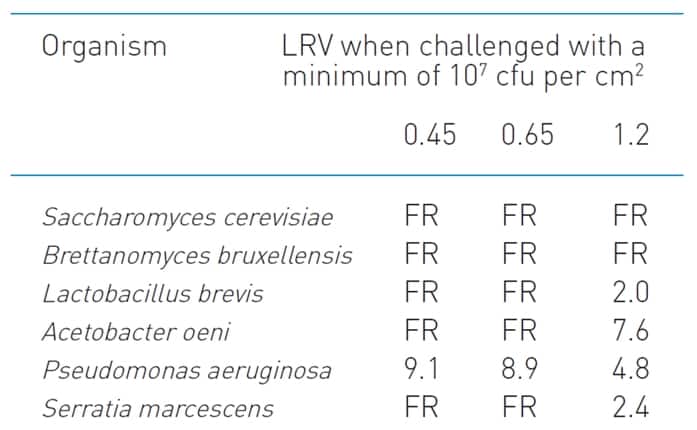
Figure 1. The data table indicating test organism sizes as specified on the Parker-domnick hunter technical data sheet for the Bevpor PH membrane.
The notation “FR” appears in several places in the table. This refers to the membrane being “Fully Retentive” under the test conditions, typically a challenge of 107 cfu (colony forming units) per cm2.
One thing that is very important to note is that there is no specific standard in Australia as to what exactly constitutes a 0.45 um wine membrane. In effect, this means that anyone can call their product a 0.45um membrane, with no defined code or specification that it must meet. That is why it is critical to always check the technical data sheet of a product before you buy it to ensure that it meets your needs in terms of retention performance in the medium you are working in (e.g. wine). The onus is on the user to verify that a membrane is suitable for the filtration specification. As they say in the classics, caveat emptor.
Filterability measurement
There are two main methods used in filterability analysis in Australia at the moment. One measures volumes of wine passed at set times, and the other measures times required to pass set volumes. The former is used extensively in New Zealand. Blue H2O Filtration recommends the latter for several reasons. Firstly, it is simple, which is always a plus in any analysis. Secondly, it generates filterability data that span a wide data range, enabling easier and more meaningful analysis. Thirdly, it is applicable to any membrane test disc, whereas the “set time” method can actually run out of sample before the completion of the assay if the wine flows fast enough, which occurs periodically with clean wines and modern membrane construction. Fourthly, it is a reliable assay, producing repeatable data.
To measure FI a sample is delivered to a membrane disc at a constant 2 bar, with the time recorded to pass 200mL and 400mL (or g, in the case of the BHF automated FI unit). A simple calculation (Bowyer & Edwards, 2014) provides the filterability index as indicated in Equation 1:
FI = T400 – 2T200 (1)
If a wine is perfectly filterable, and there is no membrane blockage, then FI 0, since T400 will be twice T200. Typically, there is some degree of blockage during the assay, and so T400 rises beyond twice T200, and so an index is generated, according to the flux decrease through the test disc.
The basic measurement of FI (Equation 1) gives a filterability snapshot of the wine up to 400 mL, but this has a limitation in that it is a relatively small measurement (0 – 400mL) and we are seeking to extrapolate this FI value to the filtration of a large volume of wine with filters of a much greater surface area. If the test is expanded from 400mL to 600mL, a modified filterability index, FIm, can be calculated. We have found that by extending the measurement to 600 mL and using 3 data points, as in Equation 2, to calculate a modified filterability index (FIm), a better understanding of the wine impact on the filtration membrane is possible.
FIm = (T600 – T200) – 2(T400 – T200) (2)
In many cases, FI and FIm are very similar values for a given wine, indicating that over time the sample is unlikely to have a deleterious effect on the filtration performance, but on occasion the two values are sufficiently different in magnitude to raise some suspicions. It may even be that FI represents a pass value (typically < 20), but FIm does not. In such cases some consideration is required, and other parameters considered, such as the condition of the wine membrane(s) being used for the job, the volume of wine to be filtered, the temperature of the wine etc.
It is important to note that filterability measurement is not a replication of the bottling train filtration. Some people question the validity of filterability data for this reason, but the reality is that the assay is not meant to reflect bottling filtration. It is simply a definitive test of wine onto membrane. If a passing filterability is measured, it is reasonable to assume that that wine will not cause filtration blockages, precisely because there are other stages of prefiltration in place on the bottling line, typically a 0.65 um membrane and a lenticular depth filter. Filterability measurement is used to screen for wines that are likely to present a problem during filtration, so that they can be prefiltered or handled alternatively so that production downtime is avoided and wine quality is maximised.
The true power of filterability measurement is knowledge, and in that respect both packager and wine producer benefit.
It is particularly important to have the correct test parameters in place when measuring filterability. Chief amongst these is that the correct membrane test disc is used, which must be identical to the wine membrane on the bottling train. Uniform temperature and homogenous samples are also important. The temperature of the sample at the time of measurement must be similar to that which will be used during bottling, otherwise the results obtained in the laboratory will not reflect what will happen during filtration at bottling.
Some dispute the necessity to have the test disc the same as the wine membrane. In the example below (Figure 2), one wine was tested with four different filterability test discs, which produced four different mass/time plots and four different sets of FI data. It is very obvious that using different test discs generates different FI data. If we stick to our usual FI pass value of 20s, only the PES test disc gives a pass result, and the fact that FI and FIm are similar suggests that the bottling run will be no problem. The cellulose acetate test discs is close, but FIm here is threefold higher than FI, so if you were using a cellulose acetate wine membrane your filters may block relatively quickly. Both nylon membrane test discs gave poor FI results, especially the 0.45um test disc. Trying to filter this wine through a nylon 0.45 um membrane would be a challenge.
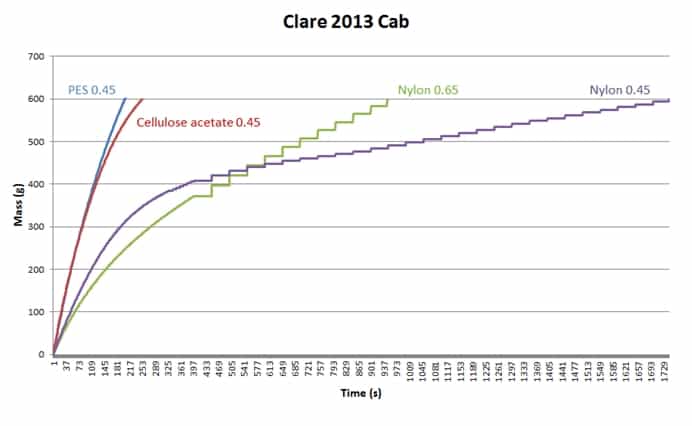
Figure 2. Filterability charts for the same wine (Clare 2013 Cabernet) measured with four different test discs.
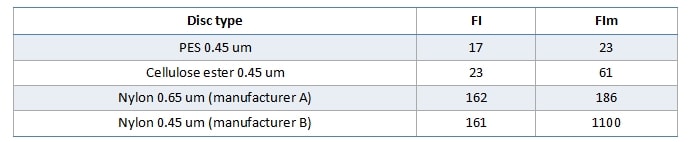
Table 1. Filterability indexes generated by the four test discs used in Figure 2.
The 0.65um test disc was included in this comparison because periodically we find people testing FI using this porosity. This is most likely a derivative of European measurements, where it is more common to see 0.60 or 0.65um membranes as the final membrane. In Australia we use 0.45um as the final membrane, and since one purpose of FI testing is to protect the final membrane, it is entirely logical to measure FI using that same membrane.
FI testing gives you no information as to the nature of the fouling component(s), only the filtration impact that the wine will have on the membrane. It can also highlight differences in membrane compositions. For example, in Figure 2 the PES membrane used is asymmetric in structure, and is able to cope reasonably well with the colloidal load of the wine. The Nylon 0.45 disc, however, is symmetrical, and consequently loads more quickly and appears to demonstrate a certain level of caking as the plot becomes more linear after about 7 minutes.
Now that we have discussed filterability analysis to some extent, let us return briefly to the topic of sterile filtration. Winemakers should be aware that the addition of concentrate just prior to packaging, whilst good practice from a microbial stability perspective, can cause filtration problems. For example, A 2014 Riesling required a concentrate addition prior to bottling of 5 g/L, which caused a significant elevation in both FI and FIm. FI rose from 8 to 53, and FIm rose from 24 to 228. These changes are shown in Figure 3 a (base wine) and b (sweetened wine). Keep in mind that the nominal threshold for a passing FI is 20. These filterability problems were identified prior to bottling due to FI analysis, yet the wine was required for scheduling reasons, and so it was sent to packaging regardless. The interesting thing in this particular case is that the prefilters on the bottling train proved their worth. A measurement of FI post-lenticulars (Becodisc 170) but pre-0.65 um membrane showed decreases in FI to 2 and 2 for FI and FIm, which was excellent news. The lenticulars were doing far more work than they should have had to, but better this than a blocked final membrane.
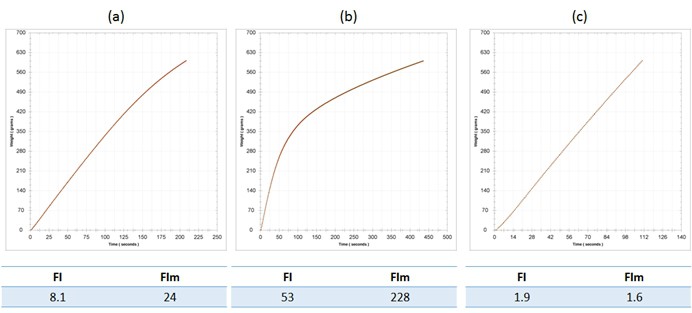
Figure 3. Filterability analysis for (a) the base wine, a 2014 Riesling; (b) the base wine with the addition of 5 g/L concentrate, and; (c) the sweetened wine post-Becodisc 170 lenticular on the bottling line. Fi and FIm are indicated for each wine stage.
Note that this was a very lucky example, for the colloidal components from the base wine and concentrate (itself a colloidal solution) addition could just as easily have passed through the lenticulars, and potentially through the 0.65um prefilter as well, blocking the 0.45 um final membrane. This can only be ascertained beforehand with significant bench filtration and filterability testing, which is not practical in most cases, hence the reliance on the direct membrane filterability test to safeguard the final membranes.
Colloids
There are two types of colloids found in wine: associative colloids and macromolecular colloids. Association colloids are aggregates of small molecules, driven to associate through weak intermolecular forces such as van der Waal’s forces, hydrogen bonding and hydrophobic interactions (Bowyer, 2003), and are typically produced by fining processes. Macromolecular colloids are comprised of one major species, such as polysaccharides, proteins, mannoproteins, carboxymethylcellulose (CMC) etc. Generally these compounds possess a molecular charge, and they can be used to stabilise association colloids, an example of which would be the addition of gum Arabic to stabilise pigmentation in reds. Colloids can present a problem for filtration in that, unless the colloid is of sufficient size to be observed by NTU, it lies latent and undetected in the wine until it is pushed through a membrane, at which point it can cause fouling. Since filterability measurement employs the membrane filtration process, the true filterability nature of a wine sample can be discerned in such a test.
In some cases wines have been cross-flow filtered and pass FI testing, only to fail a retest within a relatively short time period. We speculate that examples of this type demonstrate the impact of associative colloids, which are likely to be disrupted during cross-flow filtration through the application of shear forces, enabling a pass during FI measurement, but which then reassociate in time to once again provide a mechanism for fouling membranes. The duration of this colloidal re-association process can be from hours to months.
The impact of macromolecular colloids on FI is more straightforward, as FI can be measured pre- and post-addition to determine filterability impact. In some cases a transient elevation of FI can be observed, such as with the application of CMC (Bowyer et al., 2014).
Cross flow filtration – a cellar tool, not a bottling tool
Due to the colloidal flux imposed by cross-flow filtration, BHF recommends that cross flow filtration be used as a cellar tool rather than a pre-bottling replacement of depth filtration. As an example of colloidal changes in a wine over time post-cross flow, a 2012 Barossa Shiraz was run through a cross flow and half of the wine batch was sterile filtered within days with no problems. The second half of the wine was set for bottling two months later, with no additions or alterations made to the wine, yet when attempting sterile filtration the bottling membrane blocked. A filterability analysis (Figure 4) indicated that wine filterability was now very poor, even though the only difference from the original, filterable wine was time after cross-flow filtration. The strong red colouration of the membrane test disc (not shown) also indicated a high colloidal material level in the wine, since PES membranes typically retain very little colour (Bowyer et al., 2013). The interesting thing is that the wine was still visually unchanged and very clean at 0.55 NTU, yet filterability was poor. In such cases filterability analysis proves its worth.
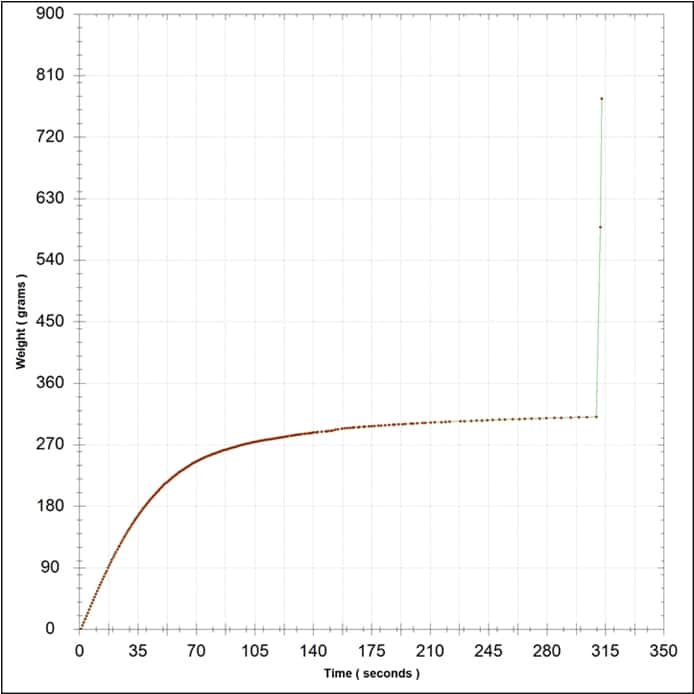
Figure 4. The filterability analysis of a 2012 Barossa Shiraz two months post-cross flow. High colloidal content was indicated by the colouration in the test disc (not shown), which correlates with the failed test result. The test was terminated due to disc blockage.
Conclusion
There is, as yet, no specific standard for filterability analysis in terms of method applied, membrane disc size and membrane composition. These parameters can influence filterability analysis outcomes, and this should be borne in mind before seeking to draw comparisons between data sets. There is also no standard for what constitutes a 0.45 um wine membrane in Australia, and we recommend close scrutiny of the technical data sheet for any membrane prior to usage in order to ascertain the suitability of the product for the task at hand. Bearing in mind the influence cross-flow filtration can have on a wine’s colloidal structure, it may prove better to treat it as a cellar tool, rather than a replacement of depth filtration during sterile filtration.
By Dr Paul Bowyer & Greg Edwards
References
Bowyer, P. (2015) Filter characteristics explained, The Australian and New Zealand Grapegrower and Winemaker, March issue (614), 64-67.
Bowyer, P. K. (2003) Molecular polarity – it’s behind more than you think, The Australian and New Zealand Grapegrower and Winemaker, November issue, 89-91.
Bowyer, P. K. and Edwards, G. (2014) Understanding filterability index: an overview and some new insights The Australian and New Zealand Grapegrower and Winemaker, November issue (610), 80-85.
Bowyer, P. K., Edwards, G. and Eyre, A. (2013) Wine filtration and filterability – a review and what’s new, The Australian and New Zealand Grapegrower and Winemaker, December issue (599), 74-79.
Gogoll, N. (2016) Top tips for filtration, The Australian and New Zealand Grapegrower and Winemaker, August issue (631), 87.
BHF wishes to thank The Australian and New Zealand Grapegrower and Winemaker for permission to reproduce this article. Subscription information can be found here.