Filterability: Critical Factors
In the March issue (674) of The Australian and New Zealand Grapegrower and Winemaker, an article was published entitled Factors Around Wine Filterability (Seabrook et al, 2020). In this article several points were made regarding membranes, filterability testing, filtration and colloidal impacts on filterability, and a sales pitch for enzymes was thrown in. All of these are valid areas of discussion when considering filtration and filterability, yet much of the information presented in this article was focused on European parameters and some outdated information, which could be very misleading for local winemakers and packaging facilities wishing to employ filterability measurement as a guide to process decisions. Some references cited in the article body also do not appear in the bibliography.
This article is presented to bring the focus back to the Aus/NZ process platform in terms of filtration and filterability, and Blue H2O Filtration has both extensive knowledge and publication bases in these areas (Bowyer & Edwards, 2018; Bowyer, 2015; Bowyer et al, 2015; Bowyer & Edwards, 2014; Bowyer et al, 2013; Bowyer et al, 2012). We recommend considering some of the following points of difference before using filterability measurement to make process filtration decisions.
Vmax
The Seabrook article (2020) introduces the terms Vmax and Clogging Index. The latter is more commonly referred to in Australia as “Filterability Index” (FI): they are identical in meaning and measurement by the method advocated by Blue H2O Filtration and we have published many articles on this topic as noted above. However, Vmax is a measurement to be very careful of. It attempts to provide a value for the viable filtration volume through a given surface area of filter. Many winemakers and packagers ask the question “How long can I expect this filter to last?” or “How many litres will this filter take?”. Both of these questions are basically the same, and pertain to Vmax, so in an ideal world the Vmax result for a given filterability test would be a great way to answer these questions, by scaling up the Vmax result according to the surface area of the filters in use.
There are significant problems with this approach, however, and for these reasons we have steered clear of using Vmax as a definitive basis for making process filtration decisions.
Firstly, Vmax is a very large extrapolation in the case of clean (low FI) wines, and it is directly proportional to the FI value. The standard filterability test (FI) requires 400 mL of wine, and Vmax attempts to use those data to generate a maximum filtration volume estimate, and this has some intrinsic error of measurement. However, the cleaner the wine, the better the filterability, the lower the filterability index and the greater the extrapolation error potential in the Vmax. In the case of wines with poor filterability the Vmax is likely to be more accurate, because the extrapolation is smaller, however this is not really useful in that such wines will typically be required to undergo cellar prefiltration, and there are other sources of error to consider.
Secondly, the filterability test (and hence Vmax) does not take into account any prefiltration extant on the packaging line, which is almost always present in the form of lenticulars and membrane prefilters, hence the packaging Vmax is always likely to exceed the measured Vmax.
Thirdly, filtration service life/capacity is an r2 law, so a doubling of filter surface area theoretically yields a four-fold increase in filter service life. In practice, and to err on the side of caution, we work on a three-fold increase in service life with a doubling of filter surface area, but this is only a rough estimate.
For these reasons it can be a dangerous game to rely on Vmax when it comes to assessing oenological treatments or filtration media, and we would caution against it.
Filterability measurement
There are two main methods used in filterability analysis in Australia at the moment. One measures volumes of wine passed at set times, and the other measures times required to pass set volumes. The former is used extensively in New Zealand. Blue H2O Filtration recommends the latter for several reasons (Bowyer and Edwards, 2018).
The methodology of FI measurement has been extensively discussed elsewhere (Bowyer & Edwards, 2018; Bowyer et al, 2015; Bowyer & Edwards, 2014; Bowyer et al, 2013; Bowyer et al, 2012), and utilises 2 data points. Rather than simply relying on the measurement of FI alone, and making process decisions on that basis, we have found that by extending the measurement to 600 mL and thus using 3 data points to calculate a Modified Filterability index (FIm), a better understanding of the wine impact on the filtration membrane is possible (Bowyer & Edwards, 2014). The calculations for both FI and FIm are quite simple (Bowyer & Edwards, 2014) and both values have meaning in assessing a wine’s filterability. BHF recommends that an FI < 20 indicates suitable filterability for sterile packaging. Confusingly, the Seabrook article (2020) terms a clogging index (viz. FI) < 50 to be “low” at one point, but then later refers to a clogging index < 20 as low. We advise caution when setting acceptable FI thresholds, and consider FI >20 to be unacceptable from a sterile packaging perspective.
Filterability testing parameters
It is particularly important to have the correct test parameters in place when measuring filterability. Chief amongst these is that the correct membrane test disc is used, which must be identical to the wine membrane on the packaging filtration train (Bowyer, 2015). Uniform temperature and homogenous samples are also important. The temperature of the sample at the time of measurement must be similar to that which will be used during packaging, otherwise the results obtained in the laboratory may not reflect what will happen during filtration at packaging. This is especially important for sweetened or carbonated wines.
The Seabrook article (2020) stipulates a 0.65 um membrane test disc, almost certainly a European derivative where it is not uncommon (though increasingly less so) to find 0.60 or 0.65 um final membranes in use in packaging. In Aus/NZ we exclusively use 0.45 um final membranes, hence the test discs used for filterability measurement must be 0.45 um, and also be identical in composition to the final membranes in packaging use. Filterability measurements made with any other test disc, whether of different porosity (eg 0.65 um) or composition (eg cellulose ester/nylon/PES) generate dubious data, and are to be avoided.
The logic here is simple. The whole purpose of filterability testing is to protect the final membranes and thus avoid unexpected and costly down time during packaging. If filterability testing employs a 0.65 um test disc, we cannot be certain that small colloids potentially in the sample will not pass through a 0.65 um prefilter and impact the 0.45 um final membrane. The only way to eliminate this possibility, and to gain a true understanding of the wine’s filterability, is to test with a membrane identical in every way to the final membrane. Anything less is basically a waste of time and money.
Some dispute the necessity to have the test disc the same as the wine membrane. In the example below (Figure 1), one wine was tested with four different filterability test discs, which produced four different mass/time plots and four different sets of FI data. It is very obvious that using different test discs generates different FI data. If we stick to our usual FI pass value of 20 s, only the PES test disc gave a pass result, and the fact that FI and FIm are similar suggests that the bottling run will present no major problem (since FIm is the longer measure). The cellulose acetate test disc gives a similar FI result, but FIm here is threefold higher than FI and significantly higher than the PES FIm, so if you were using a cellulose acetate wine membrane your filters may block relatively quickly. Both nylon membrane test discs gave very poor FI results, especially the 0.45 um test disc, in which case FIm was terrible. Trying to filter this wine through a nylon 0.45 um membrane would be a challenge.
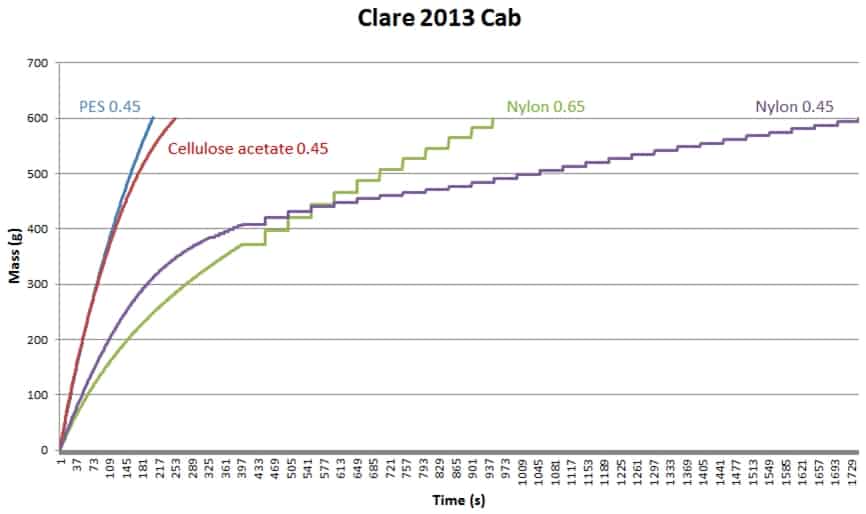
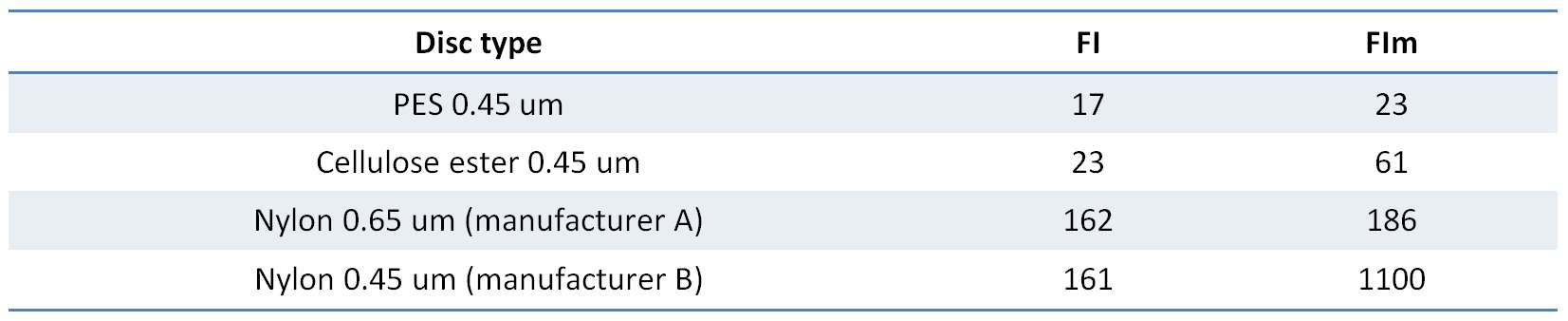
Figure 1. Filterability charts for the same wine (Clare 2013 Cabernet) measured with four different test discs.
Table 1. Filterability indexes generated by the four test discs used in Figure 1.
Enzyme additions
Enzymes are extremely useful winemaking tools. Some winemakers consider pectolytic enzymes as useful only for white fruit processing and unnecessary in reds, perhaps because the effect is visible early on in the white winemaking process as juice settles or floats nicely. To these winemakers we would argue that the same logic applies to the production of red wines, manifested in slightly different ways. Instead of juice settling, in the case of reds it is wine pressing and settling that are improved, which has the subsequent benefit of improved fruit expression in the wine.
Interestingly (depending on how nerdy you are), it is arguably wine filterability and clarity that benefit the most with the use of enzymes in red winemaking, rather than colour and tannin extraction. In support of this statement and as an example, some years ago in general discussion with Justin Bubb of Pooley Wines in Tasmania, he mentioned that he would love to bottle his Pinot unfiltered but that his final NTUs were always too high to allow this. I asked Justin if he used enzymes for red fruit processing, to which he replied in the negative, citing not wanting to change the wine’s style. After explaining that this does not really occur and that he could expect much lower final NTUs if a purified red processing enzyme was used due to improved filterability/settling/racking, he decided to give it a go. Result? Final NTUs were around 15, perfect for unfiltered bottling, and one happy customer. It is best to use purified enzymes specific for red wine production, as Brettanomyces risk and colour depletion are minimised.
The removal of pectins is what allows a red wine to press off, settle well and express fruity aromatics better. It also improves red wine filterability. One of the first tests we recommend when a red wine fails a filterability assessment is pectin and glucan tests. This can be done quickly, cheaply and easily with some ethanol and a test tube. It is not uncommon for wines destined for sterile filtration that fail filterability testing to generate a positive for the presence of pectins (Figure 2), meaning that enzymes were not used during fruit processing. The irony is that the cost of the remedial prefiltration and/or enzymatic treatment required before undertaking sterile filtration is typically far in excess of what the enzyme would have cost if used at crush, plus the additional benefits of better fruit expression/pressing/cleaner rackings etc. are not realised.
Figure 2 (below). A positive result for the presence of pectin in a red wine that failed filterability testing, as evidenced by the formation of a precipitate in the ethanol test.

Glucans, derived through Botrytis infection of the fruit or through lees maturation, have a strong capacity to foul not only membranes but also sheet filters like pads or lenticulars. This was observed extensively after the wet 2011 SA vintage, and also in subsequent vintage wines as some 2011 components were blended with later vintages. The use of glucanase enzymes (which always contain some pectolytic activity) to improve wine filterability takes time, as they are inhibited by low temperatures and the presence of tannin and ethanol. Winemakers should note that if glucans are detected via filterability testing at packaging, it is usually too late to use a glucanase enzyme to rectify the problem due to the time factors of scheduling, and alternative solutions must be found. Again, caution relying on Vmax values is advised.
Colloidal impact on membranes
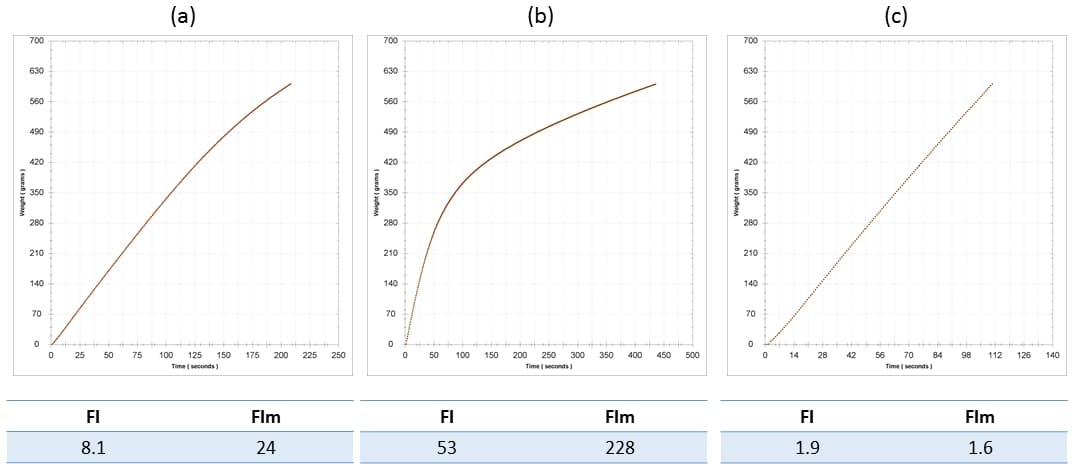
The Seabrook article (2020) states that wines exhibiting an IC (ie FI) < 20 should not experience a negative impact on filterability when further colloids are added. There are two issues here. Firstly, our experience indicates that this is often not that case, and that colloidal additions almost always increase FI, potentially with dramatic effect with respect to membrane filtration. Secondly, relying on FI alone can sometimes be misleading, in turn leading to poor process decisions, and we recommend that more data be used in filtration decision making. Perhaps one of the most commonly used colloidal additives is grape juice concentrate. As an example of what can happen in such cases, a 2014 Riesling required a concentrate addition prior to bottling of 5 g/L, which caused a significant elevation in both FI and FIm: FI rose from 8 to 53, and FIm rose from 24 to 228. These changes are shown in Figure 3a (base wine) and b (sweetened wine). Keep in mind that the nominal threshold for a passing FI is 20. These filterability problems were identified prior to bottling due to FI analysis, yet the wine was required for scheduling reasons, and so it was sent to packaging regardless. In this particular case the prefilters on the bottling train proved their worth magnificently. A measurement of FI post-lenticulars (Becodisc 170 = 0.2-0.4 um nominal) but pre-0.65 um membrane showed decreases to 2/2 for FI/FIm. The lenticulars were obviously doing more work than they should have had to, but better this than a blocked final membrane and associated replacement cost and down time, and it is always better to have the cheapest filters doing most of the work.
Figure 3. Filterability analysis for (a) the base wine, a 2014 Riesling; (b) the base wine with the addition of 5 g/L concentrate, and; (c) the sweetened wine post-Becodisc 170 lenticular on the bottling line. FI and FIm are indicated for each wine stage.
From a process perspective, this was a very lucky example, for the interacting colloidal components from the base wine and concentrate (itself a colloidal solution remember) could just as easily have passed through the lenticulars, and potentially through the 0.65 um prefilter as well, blocking the 0.45 um final membrane, necessitating a membrane changeout (including sanitisation and integrity testing) and costly down time.
Filtration efficiency nomenclature
Reference is made in the article (Seabrook, 2020) to cross-flow filters requiring “absolute membranes … to be considered a sterile filtration”. Filtration nomenclature and designation were discussed at length in a previous article (Bowyer, 2015). Such terms as “absolute” and “nominal” refer to the efficiency of a filter, but are dependent on the reference level applied. They are not relevant in membrane discussion, for the simple reason that these terms refer to a particulate testing standard, not one based on microorganism challenges. Additionally, an absolute-rated filter still falls well short of a good membrane in terms of filtration efficiency: the efficiency of membranes is typically at least 4 orders of magnitude greater than a β5000 “absolute” depth filter, hence the term “Log Reduction Value” (LRV) is used for preference. Further, whilst absolute-rated depth filters are tested against inanimate particulates of defined size, membranes are challenged with microorganisms, which can deform to fit through smaller pore sizes. Any good membrane will quote LRV’s for several organisms on its technical data sheet.
It is critical to note is that there is no specific standard in Australia as to what exactly constitutes a 0.45 um wine membrane. In effect, this means that anyone can call their product a 0.45 um membrane, with no defined code or specification that it must meet. That is why it is imperative to check the technical data sheet of a product before you buy it to ensure that it meets your needs in terms of organism retention performance in the medium you are working in (e.g. wine).
In any case, even if a suitable 0.45 um membrane for sterile filtration is used, to be termed “sterile filtered” the filter used must pass an integrity test prior to use. Integrity testing ensures that the filter was integral (ie no holes/damage etc) and the seals were effective once it was installed. Simply installing a new membrane is no guarantee of sterility in the filtrate. This is only provided by the filter passing an integrity test prior to product filtration, post-sanitisation.
Summary
Certain aspects of filterability measurement are critical for the data obtained to be meaningful in terms of process filtration decision making. The correct choice of test disc, calculation of both FI and FIm, sample temperature and homogeneity each play a role in data integrity, and these parameters must also be relevant for the Australian and New Zealand markets. We recommend that laboratories employing FI measurement ensure that they are using relevant and up to date methodology and components, to ensure that the best and most relevant data possible are obtained when seeking to make process filtration decisions based on those data.
By Dr Paul Bowyer (BHF Technologies / Blue H2O Filtration), and Greg Edwards (Vinpac International).
References
Bowyer, P. K. and Edwards, G. (2018) Filtration tips: an in-depth discussion, The Australian and New Zealand Grapegrower and Winemaker, November issue (658), 48-56.
Bowyer, P. (2015) Filter characteristics explained, The Australian and New Zealand Grapegrower and Winemaker, March issue (614), 64-67.
Bowyer, P. K. (2015) The importance of membrane choice in measuring wine filterability index, The Australian and New Zealand Grapegrower and Winemaker, March Issue (614), 68.
Bowyer, P. K., Edwards, G. and Eyre, A. (2015) Wine filtration and filterability – a review and what’s new, Petrie, P. R. ed. Efficiency and Sustainability in the Winery: proceedings of a seminar, 21-22 November 2012, Adelaide, South Australia. (Australian Society of Viticulture and Oenology, Inc.: Adelaide, South Australia).
Bowyer, P. K. and Edwards, G. (2014) Understanding filterability index: an overview and some new insights The Australian and New Zealand Grapegrower and Winemaker, November issue (610), 80-85.
Bowyer, P. K., Edwards, G. and Eyre, A. (2013) Wine filtration and filterability – a review and what’s new, The Australian and New Zealand Grapegrower and Winemaker, December issue (599), 74-79.
Bowyer, P. K., Edwards, G. and Eyre, A. (2012) NTU vs. wine filterability index – what does it mean for you?, The Australian and New Zealand Grapegrower and Winemaker, October Issue (585), 76-80.
Seabrook, A., Nazaris, B and Barthoux, J. (2020) Factors around wine filterability, The Australian and New Zealand Grapegrower and Winemaker, August issue (674), 48-50.
BHF wishes to thank The Australian and New Zealand Grapegrower and Winemaker for permission to reproduce this article. Subscription information can be found here.